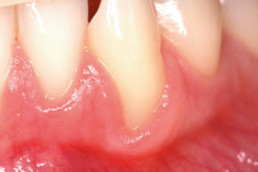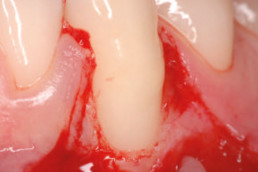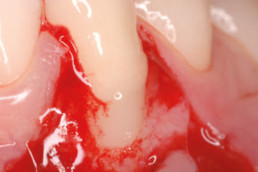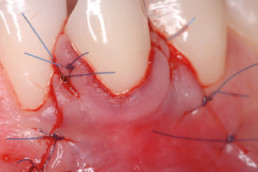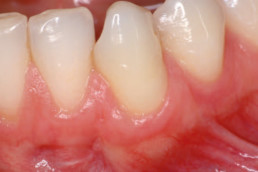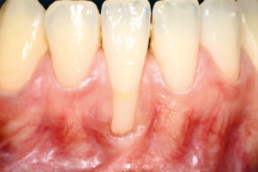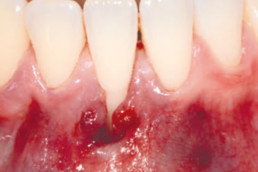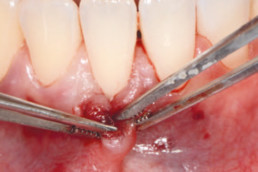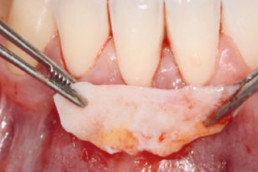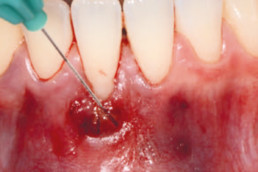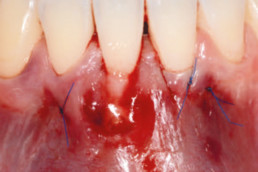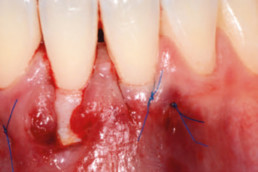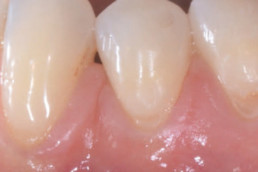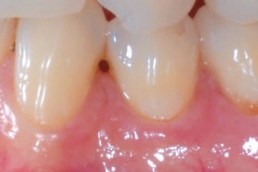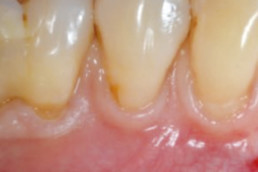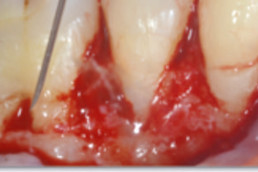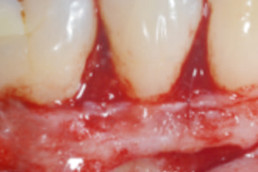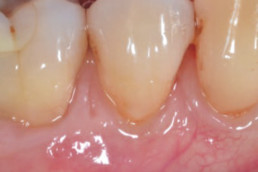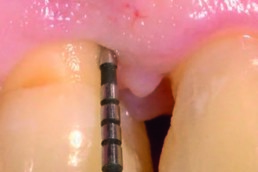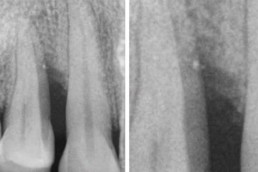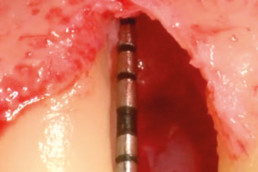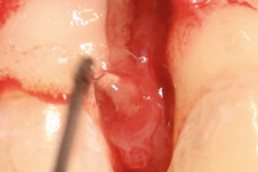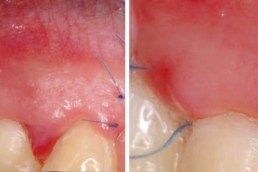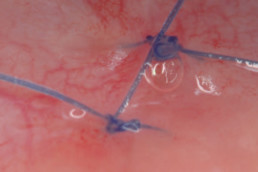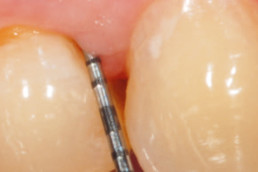
hyaDENT BG, the dental tissue regenerator for demanding dentists
Natural solution that accelerates healing and supports the regeneration of dental tissues.
Product: Tissue Regenerator
Size: 2 x 1,2ml
Composition : 1,6% cross-linked hyaluronic acid (xHyA), 0,2% native hyaluronic acid (HyA)
hyaDENT BG empowers regeneration in dental surgery
hyaDENT BG is a gel of cross-linked hyaluronic acid (xHyA), a class III medical device. It is used for soft tissue recession surgery, guided tissue regeneration (GTR) as well as guided bone regeneration (GBR). The surgical-grade highly purified cross-linked hyaluronic acid with a specially adjusted molecular weight, based on non-animal origin, is optimized for dental surgeries. The gel can be used alone in the surgical site in the presence of blood, and in combination with other biomaterials (bone graft, collagen membrane).
The pre-clinical and clinical studies confirm its properties for dental treatments:
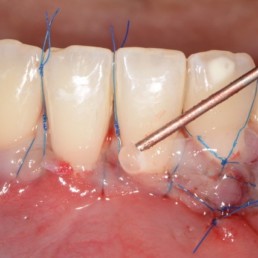
Accelerated tissue healing
Coordinates the post-operative inflammation process and accelerates neoangiogenesis. [1,2]
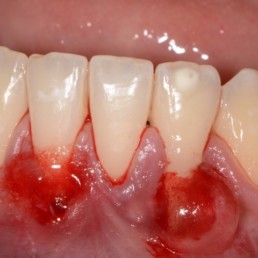
Enhanced predictability
Stabilizes coagulum and supports tissue regeneration in dental surgery. [1-4]
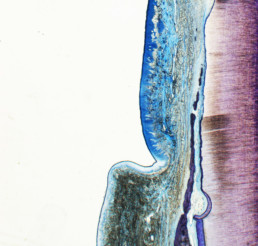
Promotes regeneration
Promotes periodontal regeneration in intrabony, recession and furcation defects. [1-4, 11]
Seven reasons to use hyaDENT BG in dental surgery

Greater outcome predictability
hyaDENT BG stabilizes blood clot and attracts growth factors to support and accelerate hard and soft tissue formation.[1-4, 17,19]

Faster tissue healing
hyaDENT BG supports angiogenesis [1,10,19] & tissue formation [12,13,15-17] over an extended period of time. Its special formulation remains present throughout the various phases of the healing process due to its slow degradation pattern (4-6 weeks).[17]

Lower risks of infection
hyaDENT BG’s bacteriostatic action reduces pathogen penetration in the surgical site. [5]

Better aesthetics
hyaDENT BG supports scar-less wound healing. [18,19]

Better patient experience
hyaDENT BG’s adjusted molecular weight reduces swelling and discomfort during the healing process. [18,19]

User friendly
Apply directly on surgical site (in presence of blood), do not rinse. hyaDENT BG can be combined with any porous bone substitute to prepare the "sticky bone" in 3 minutes.

Optimization of the properties of other biomaterials
When hyaDENT BG gel is combined with graft material hydrophilic properties are enhanced, as well as volume stability [24] and remodelling. [25] When hyaDENT BG gel is coated on a collagen membrane, its barrier effect is extended.[14]
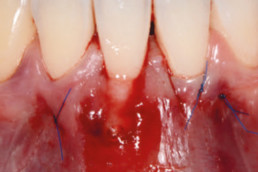
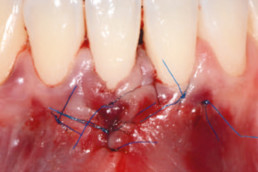
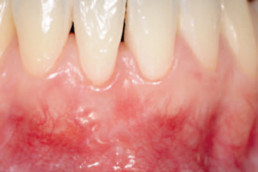
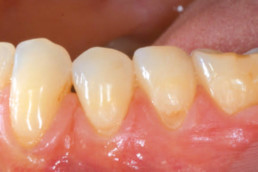
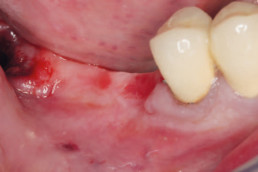
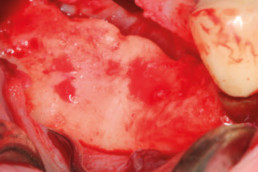
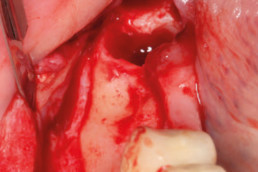
Clinical cases with hyaDENT BG
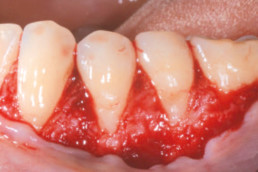
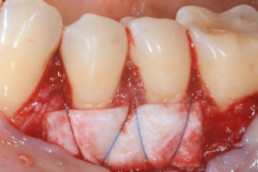
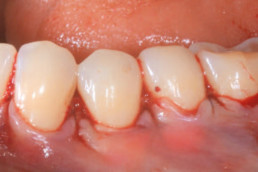
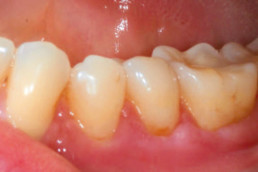
Gingival recession (CAF)
Prof. Andrea Pilloni
Gingival recession (tunnel)
Prof. Anton Sculean
Multiple recession coverage
Dr. Jürgen Pierchalla
Posterior gingival recession
Dr. Sofia Aroca
Infrabony defect
Prof. Andrea Pilloni
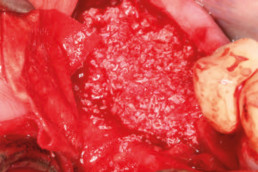
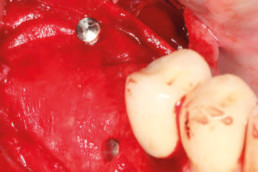
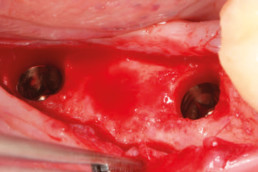
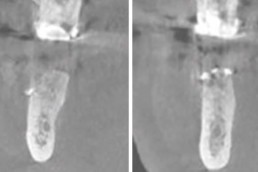
Guided bone regeneration
Prof. Darko Božić
Experts leading studies about hyaDENT BG
Handling tips
Sticky bone
Mix 1/3 of hyaDENT BG gel with 2/3 of bone graft material.
Wait for 3-5 minutes to get a stiffer sticky bone.
Mixing the sticky bone with hyaDENT BG and some blood can also contribute to get a stiffer sticky bone.
Periodontal surgery
Use blunt needle to avoid trauma on root surface (30/27G).
Clean root surface (no need to prepare e.g with EDTA).
Apply directly on wound in presence of blood (no need to work on rinsed and dry root surface).
Do not rinse the hyaDENT BG.
Add hyaDENT BG on sutured site.
Add hyaDENT BG in palatal donor site to ease healing and reduce swelling and pain.
Periodontal pocket
Ensure an effective biofilm removal prior to apply the hyaDENT BG gel.
Use blunt needle to avoid trauma on root surface (30/27G).
Do not rinse with a (CHX) mouthwash after application of hyaDENT BG.
Recent studies about hyaDENT BG
Objectives
Histologically evaluate the effects of cross-linked HA alone or combined with a collagen matrix (CM = Fibro Gide) on the periodontal wound healing/regeneration in intrabony defects.
Material and methods
Two-wall intrabony defects (5 mm wide, 5 mm deep) were surgically created at the distal and mesial aspects of mandibular premolars in six beagle dogs. The 24 defects were randomly treated as follows: open flap debridement (OFD) + HA, OFD +CM, OFD + HA+ CM (HA/CM) and OFD alone (control). At 2 months, the animals were euthanized for histological evaluation.
Results
The HA (2.43±1.25 mm) and HA/CM (2.60±0.99 mm) groups yielded statistically significantly ( P < 0.05) greater formation of new attachment (i.e., linear length of NC adjacent to newly formed bone, with inserting collagen fibers) compared with OFD (0.55±0.99 mm) group. Among the 4 treatment groups, the HA/CM group demonstrated the highest amount of regenerated tissues, although no statistically significant differences in any of the histometric parameters were observed between the HA and HA/CM groups.
Within their limits, it can be concluded that cross-linked HA alone or combined with CM
promotes periodontal wound healing/ regeneration in two-wall intrabony defects in dogs.
Conclusion
The present data have for the first time provided histologic evidence for periodontal regeneration of gingival recession defects following treatment with CAF and HA.
Background
This study evaluates the clinical outcomes of a novel approach in treating deep intrabony defects utilizing papilla preservation techniques with a combination of hyaluronic acid (xHyA – Hyadent BG) and deproteinized porcine bone mineral.
Clinical Procedure
23 patients with 27 intrabony defects were treated with a combination of hyaluronic acid (Hyadent BG) and deproteinized porcine bone mineral. Clinical attachment level (CAL), pocket probing depth (PPD), gingival recession (REC) were recorded at baseline and 6 months after the surgery.
Outcomes
At 6 months, there was a significant CAL gain of 3.65 ± 1.67 mm (p < 0.001) with a PPD reduction of 4.54 ± 1.65 mm (p < 0.001), which was associated with an increase in gingival recession (0.89 ± 0.59 mm, p < 0.001). The percentage of pocket resolution based on a PPD ≤4 mm was 92.6% and the failure rate based on a PPD of 5 mm was 7.4%.
Conclusion
The present findings indicate that applying a combined cross-linked hyaluronic acid (Hyadent BG) and xenograft approach in deep intrabony defects provides clinically relevant CAL gains and PPD reductions compared to baseline values and is a valid new approach in treating intrabony defects.
Objectives
To clinically and histologically evaluate in dogs the healing of gingival recessions treated with coronally advanced flap (CAF) with or without cross-linked hyaluronic acid (xHyA – Hyadent BG).
Material and methods
Gingival recession defects were surgically created on the vestibular side of both maxillary canines in 8 dogs. After 8 weeks of plaque accumulation, the 16 chronic defects were randomly treated with either CAF alone or CAF and cross-linked hyaluronic acid-gel (CAF/xHyA). Clinical and histological outcomes were evaluated at 10 weeks post-surgically.
Results
Compared to baseline, the clinical measurements at 10 weeks revealed a statistically significant decrease in gingival recession for both CAF (p<0.01) and CAF/xHyA (p<0.001) groups. Statistically significant differences were found in clinical attachment level (p<0.05) and width of gingival recession (p<0.01) favoring the CAF/xHyA group. Bone formation was statistically significantly greater in the CAF/xHyA group than in the CAF group (1.84±1.16mm vs., 0.72±0.62mm respectively, P<0.05). Formation of cementum and connective tissue attachment were statistically significantly higher in the CAF/xHyA group compared with the CAF group (i.e. 4.31±1.78mm versus 2.40±1.35mm and 1.69±0.98mm versus 0.74±0.68mm, respectively (P<0.05)).
Conclusion
The present data have for the first time provided histologic evidence for periodontal regeneration of gingival recession defects following treatment with CAF and cross-linked hyaluronic acid (xHyA).
Objectives
Enamel matrix derivative (EMD) in combination with flap designs aiming to maximally preserve the interdental soft tissues, is still considered the gold standard in the regenerative treatment of periodontal intrabony defects. However, increasing evidence from preclinical and clinical studies indicates that cross-linked hyaluronic acid (xHyA – Hyadent BG) possesses a number of positive biologic effects on periodontal wound healing and regeneration. However, at present, there are virtually no data from clinical studies evaluating the effects of xHyA when used in conjunction with reconstructive periodontal surgery as compared to the use of EMD. Therefore, the aim of this randomized controlled clinical trial was to compare the clinical outcomes obtained in intrabony defects following regenerative periodontal surgery using the single flap approach (SFA) in conjunction with either cross-linked hyaluronic acid or EMD.
Methodology
Thirty-two intrabony defects in 32 healthy subjects were randomly assigned: xHyA (test group) or EMD (control group). Clinical attachment level (CAL), probing depth (PD), gingival recession (REC) and bleeding on probing (BOP) were recorded at baseline,12-,18-and 24-months after surgery.
Results
At 24-months, both treatments resulted in statistically significant clinical improvements evidenced by PD-reduction and CAL-gain. The mean CAL-gain was 2.19 ± 1.11 mm in the test and 2.94 ± 1.12 mm in the control sites, respectively, without statistically significant difference between the groups. PD-reduction was statistically significantly higher for the control group (4.5 ± 0.97 mm) than the test group (3.31 ± 0.70 mm). Test sites showed slightly lower REC values (1.19 ± 0.75 mm) than the control sites (1.69 ± 0.70 mm). No statistically significant changes were observed in terms of BOP changes within and between the groups.
Conclusions
Within their limits the present findings indicate that a) both treatments led to statistically significant long-term clinical improvements, and b) cross-linked hyaluronic acid (xHyA) appears to represent a valuable alternative for regenerative treatment in intrabony periodontal defects.
Objectives
The aim of the study was to investigate the impact of two hyaluronan (HA) formulations on the osteogenic potential of osteoblast precursors.
Methodology
Proliferation rates of HA-treated mesenchymal stromal ST2 and pre-osteoblastic MC3T3-E1 cells were determined by 5-bromo-20-deoxyuridine (BrdU) assay. Expression of genes encoding osteogenic differentiation markers, critical growth, and stemness factors as well as activation of downstream signalling pathways in the HA-treated cells were analysed by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and immunoblot techniques.
Results
The investigated HAs strongly stimulated the growth of the osteoprogenitor lines and enhanced the expression of genes encoding bone matrix proteins. However, expression of late osteogenic differentiation markers was significantly inhibited, accompanied by decreased bone morphogenetic protein (BMP) signalling. The expression of genes encoding transforming growth factor-β1 (TGF-β1) and fibroblast growth factor-1 (FGF-1) as well as the phosphorylation of the downstream signalling molecules Smad2 and Erk1/2 were enhanced upon HA treatment. We observed significant upregulation of the transcription factor Sox2 and its direct transcription targets and critical stemness genes, Yap1 and Bmi1, in HA-treated cells. Moreover, prominent targets of the canonical Wnt signalling pathway showed reduced expression, whereas inhibitors of the pathway were considerably upregulated. We detected decrease of active β-catenin levels in HA-treated cells due to β-catenin being phosphorylated and, thus, targeted for degradation.
Conclusions
HA strongly induces the growth of osteoprogenitors and maintains their stemness, thus potentially regulating the balance between self-renewal and differentiation during bone regeneration following reconstructive oral surgeries.
Clinical relevance
Addition of HA to deficient bone or bony defects during implant or reconstructive periodontal surgeries may be a viable approach for expanding adult stem cells without losing their replicative and differentiation capabilities.
Objective
To clinically evaluate the healing of mandibular Miller Class I and II isolated gingival recessions treated with the modified coronally advanced tunnel (MCAT) or laterally closed tunnel (LCT) combined with hyaluronic acid (HA) and subepithelial connective tissue graft (SCTG).
Methodology
Twelve healthy patients exhibiting one isolated mandibular Miller Class I or II (Cairo Class 1) gingival recession of a depth of ≥ 3 mm, were consecutively treated with the MCAT or LCT in conjunction with HA and SCTG. Treatment outcomes were assessed at baseline and at least 6 months postoperatively. The primary outcome variable was complete root coverage (CRC).
Results
Postoperative pain and discomfort were low and no complications such as postoperative bleeding, allergic reactions, abscesses, or loss of SCTG occurred. After a mean follow-up of 18.9 ± 10 months, statistically significant (P < .0001) root coverage was obtained in all 12 defects. CRC was measured in six out of the 12 cases (50%), four cases showed a root coverage of over 95%, while the remaining two cases reached 80% and 85%. Mean root coverage was 96.09%. Mean keratinized tissue width increased from 1.6 ± 0.8 mm to 4.9 ± 1.3 mm (P < .0001) from baseline to follow-up, while mean probing depth showed no statistically significant changes (1.8 ± 0.9 mm vs 1.3 ± 0.5 mm).
Conclusion
Within their limits, the present results indicate that the described treatment approach may lead to predictable root coverage of isolated mandibular Miller Class I and II (Cairo Class 1) gingival recessions.
Objectives
To evaluate the healing of multiple adjacent type 1 and 2 gingival recessions (RT1 and RT2) treated with the modified coronally advanced tunnel (MCAT) or the laterally closed tunnel (LCT) in conjunction with a cross-linked hyaluronic acid and subepithelial palatal connective tissue grafts.
Method and materials
Fifteen healthy patients exhibiting multiple adjacent mandibular or maxillary RT1 and RT2 of a depth of ≥ 2 mm, were treated with the MCAT or LCT in conjunction with cross-linked hyaluronic acid and subepithelial palatal connective tissue grafts. Results were assessed at baseline and after a minimum of 6 months. The primary outcome variable was root coverage. Esthetic outcomes were evaluated on photographs using the root coverage esthetic score.
Results
Postoperative pain and discomfort were low and no complications occurred. Data analyses were performed at patient level. After a mean follow-up of 17 ± 5.4 months, statistically significant root coverage was obtained in all 15 cases (P < .0001). Complete root coverage was obtained in 3 out of 15 cases (20%). Root coverage amounted to > 95% in three patients, was between 90% and 95% in four patients, and reached 87.5% in another patient. In three further patients root coverage measured 75%, 77%, and 64.6%, respectively. Mean root coverage measured 85.1 ± 23.2%. Mean keratinized tissue width increased from 2.5 ± 1.0 mm to 3.7 ± 0.7 mm (P < .0001) from baseline to follow-up, while mean probing depth showed no statistically significant changes (1.3 ± 0.5 mm vs 1.5 ± 0.5 mm). The mean root coverage esthetic score was 7.9 ± 1.9, while in the three cases exhibiting complete root coverage, a maximum root coverage esthetic score (10) was given for all treated teeth.
Conclusion
Within their limits, the present results indicate that the described treatment approach may lead to predictable root coverage of multiple mandibular and maxillary RT1 and RT2.
Objective
To examine the in vitro biokinetics of hyaluronic acid (HA) from a collagen membrane (CM) and to evaluate the in vivo effect of immersion of the CM in HA solution on its degradation in streptozotocin (STZ)‐induced diabetes conditions in a rat calvaria subcutaneous model.
Background
CM degradation is accelerated in uncontrolled diabetic rats. Immersion of CM in HA has been suggested to decrease their resorption rate without interfering with their tissue integration and structural degradation. However, it is unknown to what extent CM degradation may be influenced by its immersion in HA solution under a condition mimicking a medically compromised situation with an increased inflammatory level such as diabetes.
Methodology
CMs were soaked in cross‐linked HA. Protein adsorption and the HA release were quantified by ELISA. Diabetes was induced in sixteen rats, while 16 healthy rats served as control. CM was prepared and labeled prior to implantation with Biotin. Seventeen CM were immersed in HA and 17 CM in PBS. In each animal, one test or one control disk was implanted. In order to compare the collagen content, two similar non‐implanted CM were used as baseline. Fourteen days after surgery, thirty‐two animals were sacrificed. The entire calvaria including the skin above, was chemically fixed, decalcified, and embedded in paraffin. Five‐μm‐thick sections were analyzed histologically and histomorphometrically using H&E and avidin‐peroxidase staining.
Results
The in vitro results demonstrated that the CM adsorbed roughly 80% of the total HA content. After 10 days, 36.3% of the initial HA remained on the CM. The in vivo results demonstrated that diabetes significantly reduced the thickness of the CM, while HA had a significant effect on keeping the membrane thickness. HA increased the residual collagen content in the diabetic group (P < 0.0001) but no such effect was observed in the healthy group.
Conclusion
Immersion of CM in HA prior to the implantation delays membrane degradation in uncontrolled diabetic compared with normoglycemic rats.
Objectives
To evaluate the potential added benefit of the topical application of hyaluronic acid (HA) on the clinical outcomes following non-surgical or surgical periodontal therapy.
Materials and method
A systematic search was performed in Medline, Embase, Cochrane, Web of Science, Scopus and Grey literature databases. The literature search was preformed according to PRISMA guidelines. The Cochrane risk of bias tool was used in order to assess the methodology of the included trials.Weighted mean differences (WMDs) and 95%confidence intervals (CIs) between the treatment and controls were estimated using the random-effect model for amount of bleeding on probing (BOP), probing depth (PD) reduction and clinical attachment level (CAL) gain. In order to minimize the bias and to perform meta-analysis, only randomized clinical studies (RCTs) were selected.
Results
Thirteen RCTs were included: 11 on non-surgical periodontal treatment and two on surgical periodontal treatment. Overall analysis of PD reduction, CAL gain and BOP reduction in non-surgical therapy with adjunctive HA presented WMD of − 0.36 mm (95% CI − 0.54 to − 0.19 mm; p < 0.0001), 0.73 mm (95%CI 0.28 to 1.17 mm; p < 0.0001) and − 15% (95% CI − 22 to − 8%; p < 0.001) respectively, favouring the application of HA. The overall analysis on PD and CAL gain in surgical therapy with adjunctive HA presented WMD of − 0.89 mm (95% CI − 1.42 to − 0.36 mm; p < 0.0001) for PD reduction and 0.85 mm (95% CI 0.08 to 1.62 mm; p < 0.0001) for CAL gain after 6–24 months favouring the treatment with HA. However, comparison presented considerable heterogeneity between the non-surgical studies and a high risk of bias in general.
Conclusion
Within their limits, the present data indicate that the topical application of HA may lead to additional clinical benefits when used as an adjunctive to non-surgical and surgical periodontal therapy. However, due to the high risk of bias and heterogeneity, there is a need for further well-designed RCTs to evaluate this material in various clinical scenarios.
Clinical relevance
The adjunctive use of HA may improve the clinical outcomes when used in conjunction with non-surgical and surgical periodontal therapy.
Objectives
A cemental tear (CeT) is a special type of surface root fracture that may cause periodontal and even periapical tissue destruction. Unfortunately, there is limited knowledge as to how these rare cases can effectively be treated. The present case is the first reported in the literature treating a bony defect caused by a cemental tear with hyaluronic acid (HA) and a collagen membrane. The aim of this case report is to present a regenerative surgical approach with clinical and tomographic success and stability at 2-year follow-up.
Case Presentation
A 61-year-old patient presented with spontaneous pain and gingival swelling over his right central maxillary incisor. Radiographically, a radiolucent area was observed in the medial third between both central incisors. The tomographic evaluation showed a buccal bone dehiscence and a bony defect. Once the differential diagnosis with an endodontic-periodontal lesion and root fracture was performed, CeT was the presumptive diagnosis. During the exploratory flap surgery, a small root fragment (CeT) on the mesial side of the tooth was founded and removed. The bony lesion was treated with hyaluronic acid (HA) and a resorbable collagen membrane. At 2-year follow-up clinical, radiographic, and tomographic success was observed.
Conclusion
A CeT-associated bony defect could be successfully treated after removing cemental fragments and performing a regenerative approach using HA and a resorbable collagen membrane.
Objectives
To histologically evaluate the effects of cross-linked hyaluronic acid (xHyA) with or without a collagen matrix (CM) on periodontal wound healing/regeneration in class III furcation defects in dogs.
Materials and methods
Class III furcation defects were surgically created in the mandibular premolars in six beagle dogs. The defects were randomly treated as follows: open flap debridement (OFD) + CM (CM), OFD + xHyA (xHyA), OFD + xHyA + CM (xHyA/CM) and OFD alone (OFD). At 10 weeks, the animals were euthanized for histological evaluation.
Results
The newly formed bone areas in the xHyA (4.04 ± 1.51 mm2 ) and xHyA/CM (4.32 ± 1.14 mm2 ) groups were larger than those in the OFD (3.25 ± 0.81 mm2 ) and CM (3.31 ± 2.26 mm2 ) groups. The xHyA (6.25 ± 1.45 mm) and xHyA/CM (6.40 ± 1.35 mm) groups yielded statistically significantly (p < .05) greater formation of new connective tissue attachment (i.e., new cementum, with inserting connective tissue fibres) compared with the OFD (1.47 ± 0.85 mm) group. No significant differences were observed in any of the histomorphometric parameters between the xHyA and xHyA/CM groups. Complete furcation closure was not observed in any of the four treatment modalities.
Conclusion
Within their limits, the present results suggest that the use of xHyA with or without CM positively influences periodontal wound healing in surgically created, acute-type class III furcation defects.
Abstract
This study aimed to evaluate with laser Doppler flowmetry (LDF) the effect of topical hyaluronic acid (HA) application on the vascularization of free gingival graft (FGG) donor and recipient sites during the early wound healing period and to investigate the effect of HA application on the dimensional change of the FGG. Forty systemically healthy, nonsmoking patients who required FGG due to insufficient amount of attached gingiva in a partial edentulism were randomly assigned to a study group: test (FGG+HA) or control (FGG alone). The LDF values of the donor and recipient sites were measured in both groups before the operation and at 4, 7, 10, 14, and 30 postoperative days. LDF measurement of the graft was performed as soon as the graft was taken from the palatal site. FGG dimensions (width, height, and thickness) were assessed and recorded at baseline and on day 30, as well as the percentage of the changes in these values. LDF values of the recipient site in the FGG+HA group were found to be statistically higher than those in the control group on days 4 and 7 (P = .013 and P = .020, respectively); however, no differences were found for days 10, 14, and 30. Additionally, no differences were found for the LDF values of the palatal site between the FGG+HA and control groups (P > .05) at all examined time points. The height of the graft measured on day 30 was statistically higher in the FGG+HA than the control group (P < .001). The percentage change in thickness and height of the FGG was statistically lower in the FGG+HA than control group (P = .028 and P < .001, respectively). Application of HA on the recipient bed under the FGG at the first week of healing allows the formation of a well-vascularized layer, which acts as a barrier against tissue tensions by functioning as a scaffold between the recipient bed and FGG, thus reducing the shrinkage of the graft, especially in the vertical direction. This study further showed that the graft taken from the donor site had a remaining blood perfusion value of its own.